Refractory coeliac disease - what to do when gluten free is not working
Coeliac disease is a systemic immune condition occurring in genetically susceptible individuals. An abnormal inflammatory response to dietary gluten in wheat, rye and barley results in damage to the small bowel mucosa and later villous atrophy with malabsorption. It is estimated that 1 in 70 Australians have coeliac disease and only 20% of those, have been formally diagnosed. In addition to gastrointestinal symptoms, the disease has extraintestinal manifestations, such as osteoporosis, dermatitis herpetiformis, neurological and psychological problems, liver disorders, arthritis and obstetric problems.
The only effective treatment for this lifelong disorder, at present, is a gluten-free diet (GFD) which is highly burdensome, requires long-term patient motivation, compliance and follow-up. In one study on the severity of health burden of various illnesses, the GFD was ranked second only to kidney failure with dialysis and above patients with diabetes on insulin, irritable bowel syndrome, inflammatory bowel disease, and heart failure:
"Prescribing a gluten-free diet should not be taken lightly. The diet is expensive, socially isolating and there is some evidence that questions the nutritional adequacy of a gluten-free diet when used in conditions other than coeliac disease. Given the false–positive rate with serology, commencing a strict life-long gluten-free diet is not recommended without a definite diagnosis of coeliac disease. A gastroscopy for small bowel (duodenal) biopsy is the gold standard and is recommended for all patients to confirm the diagnosis. It is generally a well-tolerated procedure with few risks." ...more information (American College of Gastroenterology Guidelines Update: Diagnosis and Management of Celiac Disease)
Some patients experience persistent symptoms due to inadvertent or deliberate gluten ingestion, a comorbid GI condition or refractory coeliac disease. Many adult patients fail to achieve complete histological healing of their small bowel mucosa despite adherence to a GFD. Several new treatments are under-development, including immunomodulators, biologics, vaccines and specific proteolytic enzymes. These should address the needs of patients with non-responsive coeliac disease with continuing symptoms and incomplete mucosal healing despite a GFD. They might also lighten the burden of a GFD in vulnerable patient groups, such as those with concomitant diabetes mellitus or neurodevelopmental/behavioural conditions. This page gives an approach when the gluten free diet seems not to be working.
What can I expect from my specialist if refractory coeliac disease is suspected?
Refractory CD is rare and existing definitions have problems. Treatments include immunomodulation with open-capsule budesonide, thiopurine and methotrexate. It is a subject of controversy whether asymptomatic patients may be labelled with refractory coeliac disease. There is not great evidence on the long term effects especially in patients that otherwise feel well. There are several steps to consider carefully:
- persistent or recurrent malabsorption symptoms (usually diarrhoea, weight loss)
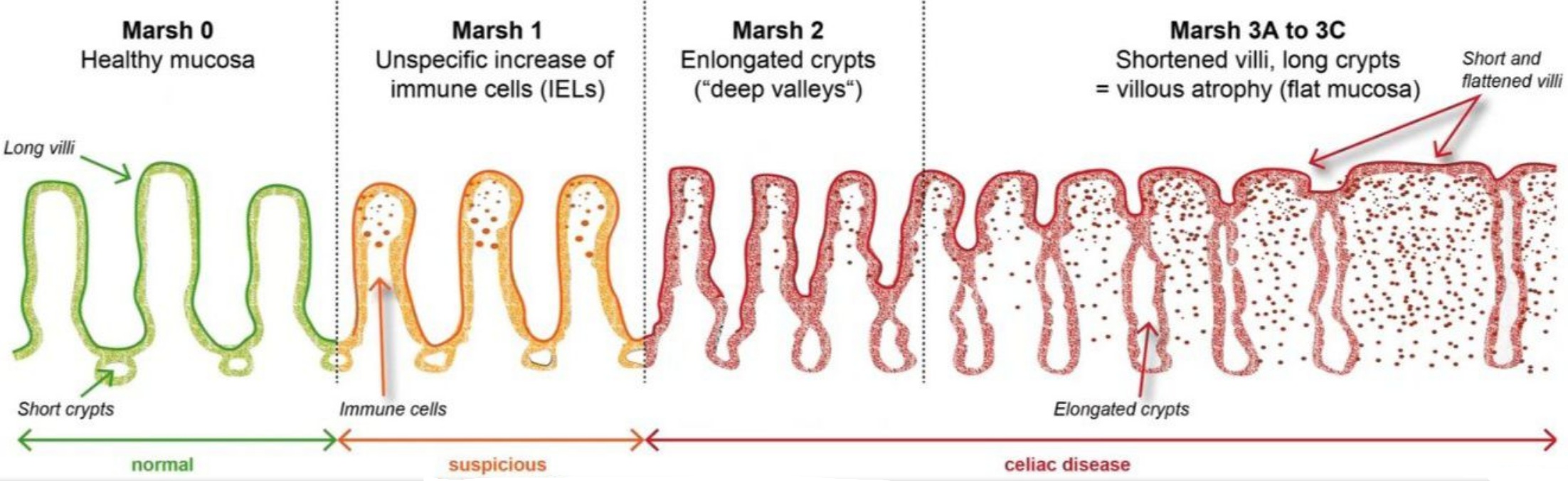
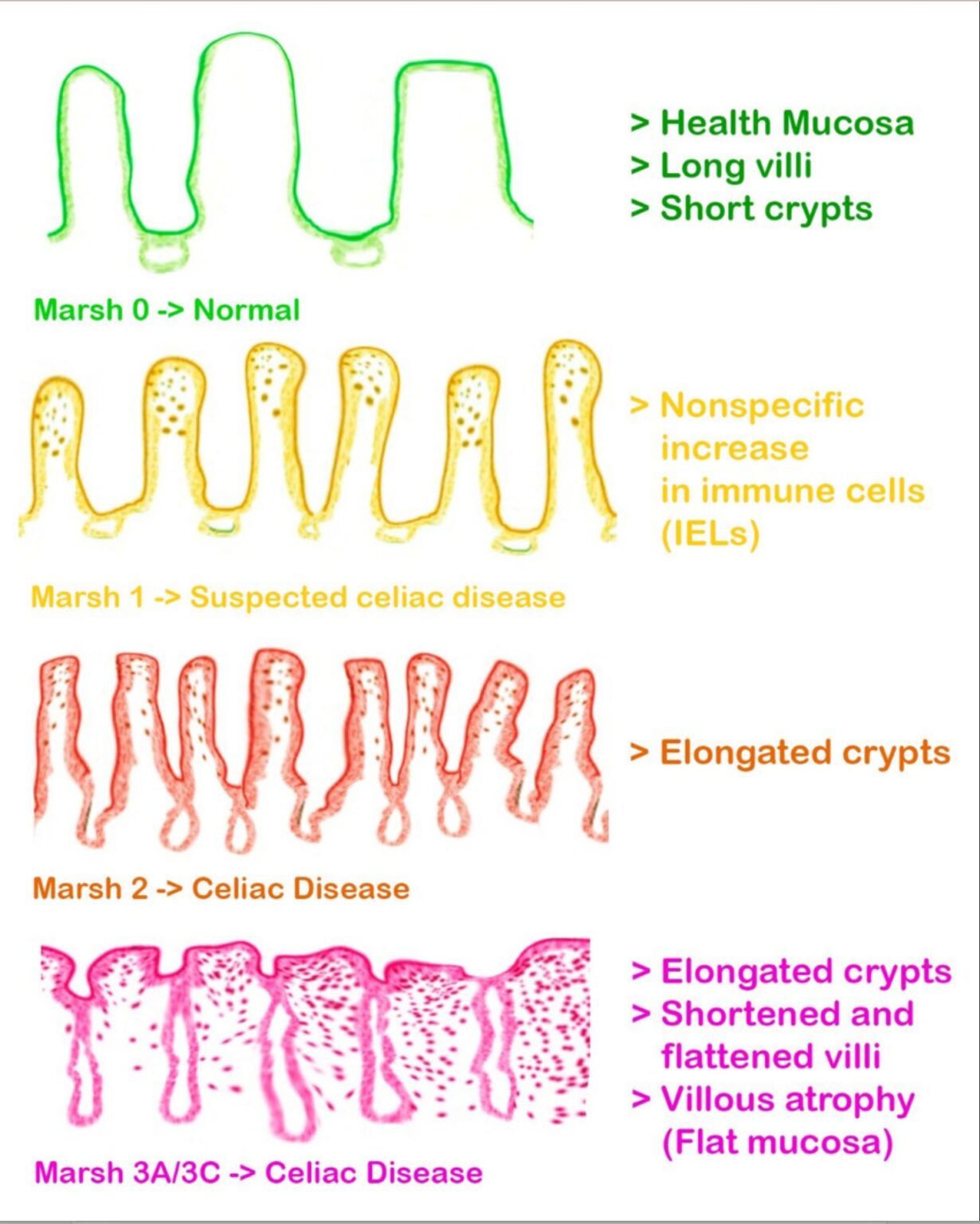
- villous atrophy (Marsh III) on biopsy despite 6-24 months of gluten free diet supervised by expert coeliac dietitian
- confirm original coeliac disease diagnosis was correct
- Consider other causes of persistent symptoms (if coeliac disease diagnosis is confirmed)
- Consider other causes of villous atrophy (particularly if coeliac disease diagnosis is in doubt)
How common is non-responsive coeliac?
In recent years, endoscopy and biopsy studies have shown that non-responsive coeliac disease includes a larger group of patients than previously thought. While current scientific literature indicates that about 30 percent of patients have non-responsive coeliac disease, there is evidence the number is closer to 50 percent when those who don’t have symptoms but continue to have damage to the intestine, called mucosal atrophy, are included. The rate of persistent villous atrophy decreases with time on the GFD, so most individuals with celiac disease may eventually have mucosal recovery. Overall, only 1/3 of adults have normal villous architecture (a healthy, healed intestine on duodenal biopsies) after 2 years on a GFD and 2/3 after 5 years on a GFD.
Major Causes of Villous Atrophy in Adults
Villous atrophy on small-bowel biopsy is a key histologic finding in several gastrointestinal disorders. While coeliac disease is the most common cause, many other conditions can produce indistinguishable or overlapping biopsy features. Accurate diagnosis requires correlation with clinical history, serology, immune status, medication use, and response to treatment.
Below is a structured, comprehensive list suitable for clinical reference or educational use.
Conditions With Pathologic Findings That Are Characteristic but Not Diagnostic
Coeliac Disease
Villous atrophy with crypt hyperplasia and increased intraepithelial lymphocytes. Diagnosis is supported by:
-
Strongly positive tTG-IgA (>10× ULN)
-
Positive EMA
-
Compatible HLA-DQ2 or HLA-DQ8
-
Biopsy-proven dermatitis herpetiformis
-
First-degree relatives affected
-
Clinical and/or histologic response to a gluten-free diet
-
Adequate sampling: ≥2 duodenal bulb + ≥4 distal duodenal biopsies
Tropical Sprue
vignette: 37-year-old aid worker, recently returned after 9 months in rural Kerala, India, with persistent foul-smelling diarrhea, weight loss, glossitis, and fatigue after a prior gastroenteritis.
- Seen in travellers or residents of South/Southeast Asia, the Caribbean, Central/South America, and West Africa.
-
Histology resembles coeliac disease
-
Treatment: Folate 5 mg daily + tetracycline 250 mg QID for 3 months (clinical response often in 2 weeks)
Adult-Onset Autoimmune Enteropathy
vignette: 45-year-old man with Hashimoto’s thyroiditis presents with months of severe watery diarrhea, steatorrhea, weight loss, and nutrient deficiencies, all unresponsive to a gluten-free diet.
-
More common in adults (~60% male)
-
Severe, persistent diarrhoea and malabsorption
-
Positive anti-enterocyte or anti-goblet cell antibodies
-
Often requires immunosuppression
-
Typically unresponsive to a gluten-free diet
Hypogammaglobulinemia / Common Variable Immunodeficiency (CVID)
vignette: 32-year-old man with recurrent sinus and lung infections now presents with chronic diarrhea, weight loss, and fatigue; mild lymphadenopathy. Stool shows Giardia
-
Low immunoglobulins, poor vaccine response; recurrent infections
-
tTG and EMA serology may be falsely negative
-
Villous atrophy with nodular lymphoid hyperplasia in some patients
Idiopathic AIDS Enteropathy
-
Occurs in advanced HIV (CD4 <100)
-
Chronic diarrhoea with villous atrophy after exclusion of opportunistic infections
2. Conditions With Pathologic Findings That Can Be Diagnostic
Eosinophilic Gastroenteritis
-
Marked eosinophilic infiltration
-
May be associated with atopy
-
Peripheral eosinophilia often present
Whipple Disease
- 45-65 Caucasian, Male, Farmer soil exposure
-
Caused by Tropheryma whipplei
-
PAS-positive macrophages in lamina propria
-
migratory large joint non erosive arthralgia years before GI, weight loss, diarrhoea, steatorrhoea+abdominal pain; neurological features (impair attention, rhythmic ocular + jaw movements), IE - culture negative
Abetalipoproteinemia
-
Fat malabsorption, steatorrhoea, acanthocytosis, neurological issues
-
Due to inability to form chylomicrons
Intestinal Lymphoma
Includes enteropathy-associated T-cell lymphoma (EATL).
-
Severe weight loss, abdominal pain
-
Consider in refractory coeliac disease
Collagenous Sprue
-
Subepithelial collagen deposition
-
Often refractory to gluten-free diet
-
May coexist with coeliac disease
Intestinal Tuberculosis
-
Granulomas on biopsy
-
Symptoms mimic Crohn’s disease
Giardiasis
-
Caused by Giardia lamblia
-
Identify via stool antigen, PCR, or trophozoites on biopsy
Crohn’s Disease
-
Patchy inflammation, possible granulomas
-
May affect proximal small bowel
3. Conditions With Non-Specific Villous Atrophy
Small-Bowel Bacterial Overgrowth (SIBO)
-
Causes mucosal injury and villous blunting
-
Improves with antibiotics
-
Trial: Metronidazole 400 mg TDS for 2 weeks (or rifaximin)
Infectious Enteritis & Parasitic Infestation
-
Numerous possible pathogens
-
Usually acute but may cause transient atrophy
Drug-Induced Enteropathy
Medications associated with villous atrophy:
-
Olmesartan (classic "sprue-like enteropathy")
-
Other ARBs
-
NSAIDs
-
Statins
-
Mycophenolate mofetil
Improves after cessation.
Severe Malnutrition
-
Seen in profound protein–calorie deficiency
-
Reversible with nutritional rehabilitation
Chronic Small-Bowel Ischemia
-
Patchy or diffuse mucosal atrophy
-
Often associated with vascular disease
Diagnostic Approach
-
Confirm true villous atrophy
Ensure multiple, adequate duodenal biopsies. -
Assess for coeliac disease
-
tTG-IgA, EMA, total IgA
-
HLA-DQ2/DQ8 if diagnosis uncertain
-
-
Review travel history
Look for risk of tropical sprue. -
Medication review
Check for sartans, NSAIDs, statins, immunosuppressants. -
Evaluate immune status
Quantitative immunoglobulins → rule out CVID. -
Screen for infections
-
Stool tests for Giardia
-
Consider HIV testing
-
-
Consider SIBO
Empirical antibiotic trial or Breath testing -
Assess for less common causes
Based on symptoms, imaging, labs (Whipple, EGE, lymphoma, Crohn’s, etc.)
Could there still be gluten in my diet?
Individual coeliac disease responses to gluten exposure are highly variable, but a chronic gluten exposure of at least 50 mg for more than a month will likely induce intestinal damage. One British gluten exposure study suggested that over a 6 month period 1/3 had intentional exposure, 1/3 had unintentional exposure and 1/3 felt they were strictly gluten free. Depending on the centre, 36-51 percent of patients with non-responsive coeliac disease are inadvertently consuming gluten.
You'll need a repeat biopsy after 6 months of a strict gluten free diet. Particularly if you have low titre tTG IgA, you'll need an expert coeliac dietitian.
- Ensure you are not consuming wheat which can be labeled as as bulgar, farina, food starch, kamut, semolina, spelt, triticale, durum.
- Recheck you are not consuming hidden gluten, ubiquitous in: toothpaste, glues, play-do, envelope or postage stamp gum, cosmetics such as lipstick and lip balms, medications and vitamins and supplements, naturally "safe" GF grains (soy, millet, buckwheat, rice and sorghum flour may have gluten-containing grains introduced during planting, harvesting, or processing), soya sauce and other sauces, fried foods, seasonings, processed meats including hot dogs and meat patties, food additives, ice cream, and even in rice cakes.
- Recheck labels of favourite everyday foods, as ingredients can change without notice
- Contact manufacturers of products that contain the statement “manufactured in a plant that also produces or used on a machine that also processes wheat” to ask about the procedures they use to avoid cross-contamination
- Recheck all over-the-counter and prescription medications with the manufacturers to be sure they do not contain gluten
- Evaluate religious ceremony/holiday foods or communion hosts to be sure they contain less than 20 parts per million of gluten
- Evaluate frequency and strategies used when dining away from home. Food preparation details matter: dusting meat with flour before grilling, using stock to cook rice, or steaming vegetables in the pasta water is not disclosed on menus.
- Ensure any ingested oats are certified gluten free and evaluate the tolerance of gluten-free oats in the diet. The addition of uncontaminated oats to the GFD has been tolerated by the majority of CD patients; however, a few people with CD may be clinically intolerant to oats
- Look for sources of cross-contamination at home, and ensure the following are implemented in the home: Use a separate toaster; Thoroughly clean kitchen counters; Use clean or separate cooking and serving utensils; Avoid “double dipping” in common condiment jars.
Additional Information
Genetic Risk and Early (Borderline) Changes
Coeliac disease only occurs in people who carry the genes HLA-DQ2 or HLA-DQ8, which are present in about 30% of the population. A positive gene test does not mean a person has coeliac disease, but a negative test makes coeliac disease extremely unlikely.
In family members of people with coeliac disease, mild early changes can occasionally be seen on biopsy, such as an increase in immune cells (intra-epithelial lymphocytes) with otherwise normal villi (no atrophy or hyperplasia). This pattern does not confirm coeliac disease but may indicate a higher risk of future diagnosis. In these cases, the patient remains on gluten unrestricted with follow-up blood tests and, at times, repeat biopsy are used to monitor for progression (tTG >10xULN+EMA+ve predicts villous atrophy and coeliac disease with >95% certainty)
Gluten Challenge (when diagnosis is uncertain)
Some people undergo testing after they have reduced gluten in their diet, which can make tests falsely appear normal. When this happens, a gluten challenge may be recommended to clarify the diagnosis. This usually involves eating the equivalent of 10–20 g of gluten per day for about six weeks (roughly equivalent to 4–8 slices of bread daily). Purified vital wheat gluten can also be used to achieve a reliable gluten dose. This is only needed in selected cases and is supervised by your doctor.
Follow-up and Monitoring
Once diagnosed, coeliac disease requires ongoing monitoring to ensure the intestine heals and nutritional issues are corrected. Follow-up may include blood tests for coeliac antibodies, checks of iron, vitamin D and B-vitamins, and occasionally a repeat endoscopy to confirm recovery. Bone density assessment may be recommended, especially in adults.
People with borderline or early biopsy findings may simply need repeat blood tests, a review of symptoms, and sometimes a repeat endoscopy after a period of observation.
Support and Resources
Living gluten-free can be challenging. Referral to a dietitian with expertise in coeliac disease can be extremely helpful. Support groups, coeliac societies and reliable online resources can also provide guidance on safe foods, dining out and managing day-to-day life on a gluten-free diet (see resources>links)
