Core IBD therapies
While newer complex therapies for IBD, represent exciting advancements, core foundational therapies like 5-ASA, prednisone, thiopurines, and methotrexate remain highly effective and are often the best choice depending on the individual case. These time-tested therapies have been rigorously studied and are supported by decades of robust evidence for their safety and efficacy.
PBS Complex Drugs Program for IBD
Inflammatory Bowel Disease (IBD) treatment has evolved to include a growing class of complex drugs, many of which target one of five key inflammatory pathways: anti-TNF agents, anti-integrins, IL-12/23 inhibitors, JAK inhibitors, and S1P receptor modulators. These therapies differ from traditional small molecules in their structure, mechanism, and site of action—often requiring advanced delivery systems or biologic manufacturing processes. While these drugs offer the promise of greater precision and efficacy, they also introduce new challenges in monitoring, sequencing, and managing long-term safety. This section explores each mechanism in practical terms, helping patients and clinicians navigate the expanding landscape of IBD therapeutics.
5 distinct mechanisms:
- anti-TNF
- infliximab (IV:®Remicade, ®Inflectra, ®Renflexis; SC:®Remsima)
- adalimumab (®Humira, ®Abrilada, ®Amgevita, ®Hadlima,®Hyrimoz; ® Adaclip, ®Hadlima, ®Yuflyma)
- golimumab (®Simponi)
- anti-adhesion: vedolizumab (®Entyvio)
- anti-IL12/23: ustekinumab (®Stelara)
- JAK inhibitors: upaticitinib (®Rinvoq), tofacitinib (®Xeljanz)
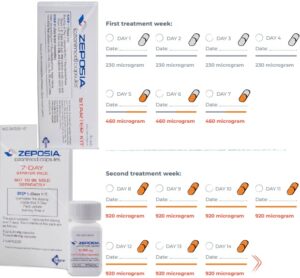
- SIP inhibitors: etrasimod (®Velsipity), ozanimod (®Zeposia)
Biologics
Small molecules
Authority applications
Services Australia's PBS Complex Drugs Programs Inquiry Line: 1800 700 270 option 6 Monday to Friday, 8 am to 5 pm:
Indications:
Ulcerative colitis ( IFX-iv, GOL, OZAN, ETRA, TOFA, UPA, USTE, VEDO-iv/sc)
- MAYO 6(or pM 6 RB2/SF2) on 3/12 5ASA+AZAT/6MP
Crohn's (ADA, IFX, UPA, USTE, VEDO)
- CDAI 300 (or 220+>50cm SI) on 6/52+AZAT/6MP/MTX 3/12
Fisulising Crohn's disease (ADA, IFX-iv/sc, UST)
Chronic pouchitis (VEDO)
- >1yr IPAA, mPDAI 5+endo 2
HPOS forms upload only needed for:
- initial treatment (UC, CD, FCD, CP)
- change or recommence (after <5 yr break) or stop (UC, CD, FCD)
- first Crohn's (CD, FCD) continuing (all)
- subsequent Crohn's (CD, FCD)continuing for agents without a biosimilar (eg. ®Remsima, UPA, USTE, VEDO)
Streamlined approval available for:
subsequent continuing treatments with PBS-subsidised biosimilar brands after first continuing application approval (see infliximab, adalimumab above)
- Crohn's (6 months)
- Fistulising Crohn's (6 months)
- Ulcerative colitis - ASUC (wk 2, 6) + UC (6 months)
Phone/HPOS approval for:
- UC - continuing treatment (non-biosimilars eg. ®Remicade, ®Humira, USTE, VEDO-IV/SC, ETRA, OZAN, GOL, TOFA, UPA)
- CD - unlisted quantity and repeats (eg. weekly adalimumab) or ®Remicade
- extended induction period - upadacitinib (8-16wks) or IV vedolizumab (14-24 wk) for Crohn's
- balance of supply applications
Toxicity severity descriptors for prednisone, thiopurines, methotrexate need to be included on forms
Dose optimisation/compassionate access programs:
- Mandatory Pharmacist for scripts/dispensing
- JustMeds Pty Ltd: PO BOX 1070 Burleigh 4220; pharmacist@justmeds.com.au
- www.justmeds.com.au
- Support: 07 2000 4124
Janssen Pro (Remicade infliximab/Stelara ustekinumab)
Pfizer - Inflectra (infliximab)
Entyvio (Vedolizumab)
Patient support programs:
Viral hepatitis
Chronic hepatitis B infection
Entecavir 500mcg 60+5 authority code:
4993 (non-cirrhotic + HBVDNA > 2,000 IU/mL or 20,000/eAg + chronic liver injury on bx/LFT);
5036 (cirrhosis CTP A+ DNA detectable + if CTP B/C -ascites, variceal bleeding, encephalopathy, alb<30, Bil>30 discuss transplant unit
NSW Health 3 year consent form 100 co-payments
Gastro-oesophageal Reflux Disease (GORD)
Pathophysiology of GORD
- LES Incompetence
- Impaired Oesophageal Mucosal Resistance
- Decreased Oesophageal Clearance
TLESR Criteria (on Manometry)
- Swallow-induced LES pressure declines >3mmHg to <2mmHg above intragastric pressure for >5s post-swallow
OR - LES relaxations >10s
1. Proton Pump Inhibitors (PPIs)
- Pantoprazole, Omeprazole, Lansoprazole, Rabeprazole, Esomeprazole
- Best for patients with overlapping dyspepsia and gastro-oesophageal reflux disease (GORD).
- Long-term use should be monitored due to potential side effects (e.g., osteoporosis, microbiome changes).
2. H2 Receptor Antagonists (H2As)
- Famotidine, Cimetidine, Ranitidine (withdrawn in some markets due to safety concerns), Nizatidine
3. Lower Oesophageal Sphincter (LES) Relaxants
- Nitrates
- Calcium Channel Blockers (Nifedipine, Amlodipine)
4. GABA(B) Agonist: Baclofen
- Short-term trials suggest reduces transient LES relaxations (TLESRs), increases LOS pressure, reduces reflux episodes, and improves reflux-related symptoms.
- Also increases gastric emptying.
- Side effects: Drowsiness, dizziness—monitor while on therapy.
5. Neuromodulation
- Tricyclic Antidepressants (TCAs): Amitriptyline, Nortriptyline (Nortriptyline preferred if drowsiness is a concern).
Prokinetics: An Overview
Prokinetic agents are medications designed to enhance gastrointestinal motility by accelerating gastric emptying. However, the relationship between their physiological effects and symptom relief remains complex. This document explores the challenges and considerations in prokinetic therapy, available treatment options, and the evolving approach to managing motility disorders.
Symptom Response vs. Gastric Emptying
- There is limited correlation between the acceleration of gastric emptying and symptom relief, suggesting that factors beyond measurable gastric motility play a role in patient outcomes.
Multiple Contributing Factors
- Symptoms in conditions like gastroparesis, functional dyspepsia, and irritable bowel syndrome (IBS) are influenced by complex mechanisms beyond gastric emptying alone.
Safety Concerns with Prokinetics
Effective but non-selective agents have been abandoned due to intolerable side effects:
- Cholinergics: Caused significant bladder spasms.
- Cisapride: Linked to cardiac arrhythmias (Torsades de Pointes, ventricular tachycardia), leading to its withdrawal.
- Tegaserod: Raised concerns regarding cardiac safety.
Drug Development Constraints
- A strict "safety-first" policy for non-life-threatening conditions limits the development of new drugs for debilitating motility disorders such as gastroparesis and intestinal pseudo-obstruction.
Potential Benefits of Multi-Targeted ("Dirty") Drugs
While highly selective agents are preferred to minimize adverse effects, some multi-target drugs may provide enhanced symptom relief:
- 5-HT4 agonist & 5-HT3 antagonist combinations: May be beneficial in IBS.
- Linaclotide: Primarily a pro-secretory agent, but also has unexpected pain-relieving effects in constipation-predominant IBS.
- Prucalopride: A highly selective 5-HT4 agonist with potential neuroprotective effects relevant to enteric neuropathic disorders.
QT Prolongation Risk Across Drug Classes
Many commonly used medications can prolong the QT interval, necessitating careful patient monitoring. These include:
- Antihistamines: Diphenhydramine
- Antidepressants: Amitriptyline, citalopram, mirtazapine
- Antipsychotics: Haloperidol, quetiapine
- Macrolide antibiotics: Erythromycin, azithromycin
Comprehensive Management Approach
Given the multifactorial nature of motility disorders, an integrative approach addressing nausea, pain, gastric function, and psychological health may improve outcomes.
Differential Diagnoses
Conditions that may mimic gastroparesis or other motility disorders include:
- Peptic ulcer disease, gastric cancer, coeliac disease, abdominal angina.
- Anastomotic ulcers, internal herniation, gallbladder disease (for early dumping syndrome).
- Insulinoma or surreptitious use of glucose-lowering medications (for late dumping syndrome).
Prokinetic Agents and Related Therapies
Cholinergic Agents
- Neostigmine (muscarinic M2 agonist) – Used in Ogilvie’s syndrome.
- Pyridostigmine – Used for pseudo-obstruction.
Dopamine Antagonists
- Metoclopramide (DA, 5-HT3/4 antagonist, mild anticholinesterase activity) – Side effects: fatigue, sleepiness, depression, akathisia, anxiety, dyskinesia.
- Domperidone (DA2 antagonist) – 10–20 mg QID; requires cardiac monitoring due to potential QT prolongation.
Serotonin Agonists
- Mosapride (selective 5-HT4/3) – Minimal QT prolongation risk, common side effects include mild GI disturbances.
- Prucalopride (highly selective 5-HT4 agonist) – Greater efficacy with minimal cardiac risks.
- Mirtazapine (tetracyclic antidepressant, 5-HT1A/2A/3 antagonist) – May contribute to fundus relaxation, antiemetic, and anxiolytic effects.
Macrolide Antibiotics (Motilin Receptor Agonists)
- Erythromycin (IV: 3 mg/kg every 8 hours; Oral: 250 mg TID) – Risk of tachyphylaxis; use at lower doses may prolong efficacy.
- Azithromycin – Similar efficacy with less QT prolongation.
Other Treatments
- Botulinum Toxin (Botox®) – Occasionally used for pyloric dysfunction.
- Complementary Therapies – Ginger, peppermint, Iberogast, melatonin, and capsaicin may offer symptomatic relief in functional dyspepsia.
- 5-HT3 Antagonists – Ondansetron (4–8 mg TID PO, IV, PR), granisetron.
- Phenothiazines – Prochlorperazine (Stemetil®) for nausea.
- Antihistamines – Cyclizine, diphenhydramine, and cyproheptadine (Periactin™).
- Neurokinin-1 Receptor Antagonists – Aprepitant (125 mg daily).
- Anticholinergics – Scopolamine patch.
- Benzodiazepines – Lorazepam (used in chemotherapy-related anticipatory nausea).
Conclusion
While prokinetic therapy plays a key role in managing gastrointestinal motility disorders, challenges remain due to limited symptom correlation, safety concerns, and regulatory constraints. A balanced approach, integrating pharmacologic and non-pharmacologic strategies, is essential for optimizing patient outcomes. Future research may identify novel agents that offer enhanced efficacy with improved safety profiles.
Abdominal/Chest Pain Modulation
- Psychosocial Therapies
- Strongly indicated for severe pain, impaired quality of life (QOL), and mood/anxiety disorders.
- Options include:
- Cognitive Behavioral Therapy (CBT): Proven to help in functional gastrointestinal disorders (FGIDs).
- Gut-Directed Hypnotherapy: Effective for visceral hypersensitivity and functional dyspepsia (FD).
- Biofeedback: Useful for stress-related GI symptoms.
- Social Support: Social workers may assist in workplace/home/school adjustments.
- Neuromodulators (for Visceral Hypersensitivity & Functional Dyspepsia)
- Tricyclic Antidepressants (TCAs): Amitriptyline, nortriptyline (helpful even at low doses).
- Mirtazapine: Particularly useful for nausea and weight loss.
- Gabapentinoids (Gabapentin, Pregabalin): Reduce visceral pain sensitivity, may be beneficial in patients with FD and early satiety.
- Serotonin Antagonist
- Cyproheptadine: Particularly beneficial for postprandial distress syndrome (PDS) subtype, especially in younger patients with early satiety.
- Proton Pump Inhibitors (PPIs)
- Pantoprazole, Omeprazole, Lansoprazole, Rabeprazole, Esomeprazole
- Best for patients with overlapping dyspepsia and gastro-oesophageal reflux disease (GERD).
- Long-term use should be monitored due to potential side effects (e.g., osteoporosis, microbiome changes)
Management of Abdominal Bloating
1. Antifoaming Agents & Surfactants
- Simethicone: Alters gas bubble elasticity, facilitating gas passage.
- Activated Charcoal: May help reduce gas formation and odour.
2. Prokinetics
- Erythromycin (low-dose): Motilin agonist, accelerates gastric emptying.
- Metoclopramide: Dopamine antagonist, enhances gastric motility (long-term use may cause tardive dyskinesia).
3. Antibiotics for Suspected Bacterial Overgrowth
- Rifaximin: Poorly absorbed, highly effective against both aerobic and anaerobic bacteria in small intestinal bacterial overgrowth (SIBO).
- Metronidazole (Flagyl®) and Trimethoprim/Sulfamethoxazole (Bactrim®): Alternative options if Rifaximin is unavailable or ineffective.
4. Probiotics
- Bifidobacterium infantis (35624 strain):
- Demonstrated positive effects in randomized controlled trials (RCTs).
- Reduces intestinal inflammation and improves symptoms of pain, bloating, and bowel movement difficulties compared to placebo.
- Other probiotic strains:
- Lactobacillus GG and Saccharomyces boulardii may also be beneficial in select cases.
5. Digestive Enzymes
- Lactase: Useful for lactose intolerance.
- Alpha-galactosidase (Beano): Helps break down fermentable carbohydrates to reduce bloating.
- Pancreatic enzyme supplements: Beneficial in pancreatic insufficiency to aid digestion.
6. Aperients (Laxatives) for Constipation-Related Bloating
A. Laxatives Less Likely to Cause Bloating
- Osmotic Laxative: Polyethylene Glycol (PEG/Miralax) → Softens stools with minimal bloating.
- Bulk-Forming Laxative: Methylcellulose (Citrucel) → Non-fermentable, less likely to cause gas.
- Stimulant Laxative: Senna (low dose) → Effective for short-term relief of constipation-related bloating.
- Natural Option: Kiwi Fruit → Increases bowel movement frequency with low bloating risk.
B. Laxatives That May Worsen Bloating
- Lactulose & Magnesium Hydroxide: Can cause gas and bloating due to fermentation.
- Psyllium (Metamucil): Fermentable fiber, can increase bloating if not taken with sufficient water.
- Prunes (Dried Plums): Contain sorbitol and fructans, which may cause bloating in some individuals.
7. Dietary Approaches
- Low FODMAP Diet:
- Well-established for reducing bloating and IBS symptoms.
- Elemental Diet (for severe SIBO cases):
- Reduces fermentation and supports gut healing.
8. Herbal & Alternative Therapies
- Peppermint Oil (Enteric-Coated):
- Works as an antispasmodic, reduces bloating.
- Clinical trials show benefits in IBS and functional dyspepsia.
- Iberogast (Combination of Herbal Extracts):
- Includes chamomile, peppermint, licorice root.
- Helps functional dyspepsia, bloating, and motility disorders.
- Ginger Extract:
- Stimulates gastric emptying, reduces nausea and bloating.
Diet:
gastroparesis (Postprandial fullness, early satiety, bloating, abdominal distension, nausea, and vomiting, abdominal pain, and dysphagia): "fork mashable"foods (low particle size diet), Low fat, low fiber foods small frequent meals
anti-dumping diet if rapid GE (
| Early dumping syndrome (within 1 h after meal ingestion Gastrointestinal symptoms: Abdominal pain, epigastric fullness, diarrhea, nausea, vomiting, borborygmi, and bloating Vasomotor symptoms: desire to lie down, palpitations and tachycardia, fatigue, faintness, syncope, perspiration, headache, light-headedness, hypotension, flushing, and pallor |
| Late dumping syndrome (1–3 h after meal ingestion) Neuroglycopenia: fatigue, weakness, confusion, hunger and syncope Autonomic reactivity: perspiration, palpitations, tremor and irritability |
(20% of patients suffer from symptoms of dumping syndrome after vagotomy and pyloroplasty, 40% after Roux-en-Y bypass and sleeve gastrectomy, and peak at 50% after esophagectomy): Eat small, frequent meals at least 6 times a day; Lie down as soon as you finish eating. Avoid: simple sugars such as sweets,high sugar soft drinks, cakes, and cookies. Avoid foods that are very hot or very cold. . These can trigger dumping syndrome symptoms. Do not drink liquids with your meal. Instead, drink liquids at least a 30 minutes to an hour after eating solid food. Encourage high-fibre, high-protein food
Prunes. Dried plums (prunes) not only contain fiber but also sorbitol and fructans, non-absorbable carbohydrates that, when fermented by colonic bacteria, create an osmotic load that can dramatically alter stool frequency and consistency. In an 8-week single-blind, randomized study with 40 constipated subjects, the number of CSBMs per week and stool consistency scores improved significantly (P < 0.05) with prunes when compared to psyllium. Straining and global constipation symptoms did not differ significantly between treatments.87
Kiwi. In a recent study from Asia, 41 IBS-C patients and 16 healthy adults consumed 2 Hayward green kiwifruits per day for 4 weeks. Another 13 IBS-C patients served as controls. IBS-C patients that consumed kiwi fruit had a significantly faster colonic transit time than controls (P = 0.026). The IBS-C kiwifruit group also reported increases in defecation frequency and improvements in bowel function
Safe prescription of drugs which prolong the QTc interval
Drug-induced QTc prolongation is not a universal phenomenon. Why some individuals are susceptible to this condition and others are not, is still unclear. They may possibly have a subclinical genetic mutation that is only revealed when they are exposed to certain drugs. Before prescribing a drug that is known to cause QTc prolongation, it is important to enquire about any past history of syncope or cardiac arrest. Also obtain a detailed family history of syncope, sudden death at a younger age or congenital deafness5 (a feature of Jervell and Lange-Nielsen syndrome). Any suspicion of a congenital long QTc syndrome should be confirmed with a 12 lead ECG. If the ECG shows prolongation of the QTc interval, drugs which could make it worse should be avoided.
Co-administration of two or more implicated drugs or an offending drug with a substance capable of inhibiting its hepatic metabolism should be avoided. It is important to question the patient about the consumption of non-prescription medications (such as terfenadine and astemizole) before prescribing a drug which can prolong the QTc interval. An association with a medication that prolongs the QTc interval should be sought in patients who present with syncope or cardiac arrest. Such a relationship should particularly be looked for in patients with no cardiac history or relevant family history.
When an implicated drug is prescribed to a high-risk patient (Table 1), it is advisable to perform a 12 lead ECG within the first few days of treatment to look for QTc prolongation beyond normal limits. If QTc prolongation is observed, it is advisable to stop the offending drug or switch to an alternative drug that has no such effect.

_PG_1.jpg)